Что такое алмаз
. COMPOSITION | CRYSTAL STRUCTURE | HARDNESS | DURABILITY
Diamond is carbon in its most concentrated form. Except for trace impurities like boron and nitrogen, diamond is composed solely of carbon, the chemical element that is fundamental to all life.

But diamond is distinctly different from its close cousins the common mineral graphite and lonsdaleite, both of which are also composed of carbon. Why is diamond the hardest surface known while graphite is exceedingly soft? Why is diamond transparent while graphite is opaque and metallic black? What is it that makes diamond so unique?
The key to these questions lie in diamond’s particular arrangement of carbon atoms or its crystal structure—the feature that defines any mineral’s fundamental properties. A crystal is a solid body formed from the bonding of atomic elements or compounds in a repeating arrangement. Often, crystals possess smooth external faces. Due to their symmetrical and finite nature, the building blocks of crystals are limited to relatively small numbers of atoms, and their chemical compositions to simple numerical combinations of elements.
A neutral carbon atom has 6 protons and 6 electrons surrounding its nucleus. Four of the electrons in a carbon atom are valence electrons, which are electrons that are available to form bonds with other atoms. In graphite, each carbon atom bonds only 3 of its 4 valence electrons with neighboring carbons. The resulting structure of these bonds is a flat sheet of connected carbon atoms. Though individually strong, these layers are only weakly connected to one another, and the ease with which they are separated is what makes graphite so slippery.

This model shows how each carbon atom (ball) is connected to 4 other carbon atoms by strong chemical bonds (rods), creating diamond’s rigid crystal structure.
In diamond however, every carbon shares all 4 of its available electrons with adjacent carbon atoms, forming a tetrahedral unit. This shared electron-pair bonding forms the strongest known chemical linkage, the covalent bond, which is responsible for many of diamond’s superlative properties. The repeating structural unit of diamond consists of 8 atoms which are fundamentally arranged in a cube.
Using this cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop in a variety of different shapes known as «crystal habits.» The octahedron, or eight-sided shape that we associate with diamonds is its most common crystal habit. But diamond crystals can also form cubes, dodecahedra, and even combinations of these shapes. All of these shapes are manifestations of the cubic crystal system to which the mineral diamond belongs. Two exceptions are the flat form called a macle, which is actually a composite crystal, and etched crystals, which have rounded surfaces and, sometimes, elongated shapes.

These idealized drawings show some of the common crystal habits of diamond. Clockwise from left to right they are an octahedron, a cubo-octahedron (a combined form), a dodecahedron, a macle twin, and a cube.
Real diamond crystals don’t have completely smooth faces. Trigons are triangular growths that reflect subtle changes in height on a diamond’s face. The trigons shown here are slight indentations that were most likely produced by a natural etching of the crystal. However, raised trigons, which point in the same direction as the crystal face, may also occur from etching, dissolution, or as part of the natural growth of the crystal.

This image of trigons was created with Nomarski differential interference contrast microscopy and is 0.29 mm across. Photo by John I. Koivula courtesy of the Gemological Institute of America.
Diamond is renowned for its hardness. Hardness is the measure of a substance’s resistance to being scratched, and only a diamond can scratch another diamond. Diamond is the hardest substance known.
The Mohs scale—a hardness scale developed in 1822 by Austrian Friedreich Mohs as a criterion for mineral identification—can help us appreciate the hardness of diamond. The scale ranks 10 minerals; harder minerals, with a higher number, can scratch those with a lower number.
When the mineral hardness numbers from the Mohs scale are plotted against those on the more quantitative Knoop scale (based on the force needed to make indentations using a diamond), we can see how it doesn’t adequately express the extreme hardness of diamond. The Mohs scale is relatively stable until it reaches the eighth mineral topaz, but it jumps exponentially from corundum (colorless sapphire) to diamond. It is in fact difficult to measure the hardness of diamond, because diamond must be used to measure its own hardness.

Hardness is not the only measure of a mineral’s durability—the relative resistance to fracture is another. Although diamond is not fragile or prone to breaking apart, all substances including diamond can fracture or shatter. Due to its particular crystal structure, diamond has certain planes of weakness along which it can be split. Diamond is said to have perfect cleavage in four different directions, meaning it will separate neatly along these lines rather than in a jagged or irregular fashion. This is because the diamond crystal has fewer chemical bonds along the plane of its octahedral face than in other directions. Diamond cutters take advantage of cleavage to fashion diamonds efficiently.
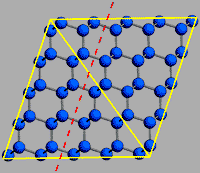
The cleavage direction represents a layering in the structure of diamond—there are fewer bonds over a given distance across the layers than within them. This drawing shows the cleavage direction with a dashed red line.




